International collaborations
We collaborate with North American and European databases and experts in multi-country database studies under the principles of the European Network of Centres for Pharmacoepidemiology & Pharmacovigilance (ENCePP). We are dedicated to excellence in research by adhering to the ENCePP Guide on Methodological Standards and promoting scientific independence and transparency. We have registered studies in the HMA-EMA Data Catalogue, a publicly accessible resource for the registration of pharmacoepidemiological and pharmacovigilance studies.
Strengths of the local data partner model
- Trusted long-standing working relationships – familiarity, shared collaboration platforms, ‘short-hand’ in communications & project management
- Reliance on experts who know the local health system, care management practices – and thus the nature of the data, allows ‘short-cutting’ the feasibility and study adaptation process
- Delivers global reach with navigation through a single point-of-contact who you can trust to ensure study quality

SIGMA Consortium
The SIGMA Consortium is a contract-based alliance of ENCePP research centers with proven institutional pharmacoepidemiologic and real-world evidence (PE/RWE) experience, providing a one-stop shop for research services. It is the best ‘go-to’ organization for comprehensive and rigorous methodological research, launched to enable multi-country studies that use common analyses to study responses to treatments in large populations.
Specifically, the SIGMA Consortium conducts the following activities:
- Act as a trusted hub for the planning, design, contracting, analyses, delivery, interpretation and dissemination of European PE/RWE studies, providing a one-stop shop to entities that request research services
- Provide training and education through participation in studies
- Promote, conduct, and facilitate innovative methodological research
The vision of the SIGMA Consortium is to provide best evidence on the use, benefits, and harms of medications, vaccines, and devices to improve public health and patient care. Its mission is to provide trusted PE/RWE through a European federated professional network of excellence based on the ENCePP Code of Conduct.
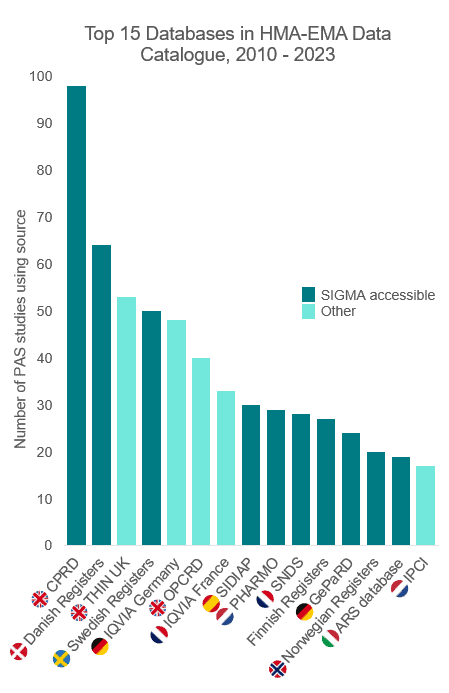
Sources accessible through the SIGMA Consortium comprise 10 of the top 15 secondary databases used in European PASS[i]
[1] Chioureas D, Bjornfors R, O’Connell S, et al. Real-World Evidence in post-authorization studies: Review of European databases in the ENCePP EU PAS register® between 2010 – 2023. Presented at ISPOR EU, Copenhagen, 13-16 November 2023. https://ispor-sf13.matrixdev.net/heor-resources/presentations-database/presentation/euro2023-3788/129959
